Supplement New Guide: Explore Innovations for 2025
The supplement industry is experiencing a surge of innovation as we approach 2025. From groundbreaking ingredients to advanced delivery systems, supplement new trends are transforming how consumers support their health.
This comprehensive guide explores the supplement new landscape, highlighting the latest ingredient breakthroughs, technology-driven solutions, and regulatory updates.
Whether you are a supplement user, industry professional, or health-conscious individual, you will find actionable insights to help you make informed choices. Stay ahead of the curve and discover what the future holds for supplements in 2025.
The Evolving Landscape of Supplements in 2025
The supplement new landscape in 2025 is more dynamic than ever before. Global demand for wellness products is fueling both innovation and diversification across the market. Consumers are no longer satisfied with generic multivitamins. They seek targeted solutions for specific health goals, ranging from immune support to longevity.
Aging populations and a growing focus on preventive wellness have become major drivers of supplement new development. People want to maintain vitality and quality of life as they age, which pushes brands to create more specialized products. Additionally, the pandemic’s impact continues to reshape priorities, placing greater emphasis on immune health, mental well-being, and overall resilience.
Market Snapshot: Supplement Industry 2025
| Metric | 2023 Value | 2025 Projection |
|---|---|---|
| Global Market Size (USD) | $163 billion | $190 billion |
| Growth Rate (CAGR) | 7.5% | 8.2% |
| Leading Segments | Vitamins, Probiotics, Plant-Based | Personalized, Science-Backed, Digital Integration |
Consumers are increasingly prioritizing safety, transparency, and personalization in their supplement new purchases. Labels now highlight third-party testing, clean ingredients, and traceable sourcing. This demand for information is prompting brands to share more about their research and supply chains.
The supplement new industry is also seeing deeper collaboration between medical professionals and product formulators. Physicians, nutritionists, and researchers are working alongside supplement companies to develop formulas that address real clinical needs. This trend is improving both safety and efficacy, making supplements a more trusted part of health routines.
Global events have accelerated these changes. The post-pandemic era has made consumers more proactive about health, leading to a surge in adaptogens, immune boosters, and mental wellness supplements. Companies are responding with flexible production models and rapid product launches.
Regulatory bodies like the FDA are stepping up educational initiatives and hosting public meetings to promote responsible supplement new innovation. These efforts help ensure that new products meet safety and efficacy standards, while also informing both industry professionals and the public.
One of the most significant shifts is the rise of science-backed, evidence-based supplement new products. Companies are investing in robust clinical studies and transparent ingredient sourcing. For a closer look at the latest science-backed supplement ingredients, explore comprehensive resources that outline the research and benefits behind today’s leading compounds.
As 2025 unfolds, the supplement new market will continue to evolve through a blend of cutting-edge research, consumer empowerment, and regulatory guidance. Staying informed is essential for making smart, safe choices in this rapidly changing industry.

Regulatory Updates and Compliance: What’s New for 2025?
The supplement new landscape in 2025 is being shaped by rapid regulatory evolution and heightened compliance standards. As the industry expands, agencies like the FDA and FTC are intensifying oversight to ensure consumer safety and product legitimacy.
Global harmonization efforts are also on the rise, with international agencies collaborating to align supplement new standards. Businesses and consumers must remain vigilant, as recent guidance and enforcement actions are reshaping how supplements are formulated, labeled, and marketed.

New Ingredient Notification and Master Files
A major focus for supplement new products in 2025 is compliance with the FDA’s New Dietary Ingredient (NDI) Notification Process. This process requires manufacturers to notify the FDA before introducing new ingredients not previously used in supplements prior to 1994. The June 2025 updates emphasize stricter documentation and scientific evidence, ensuring that supplement new formulations meet rigorous safety standards.
Master files play a vital role by protecting proprietary data while supporting transparent ingredient review. These confidential files allow companies to share critical safety data with the FDA without revealing trade secrets to competitors. The FDA’s educational materials, released in June 2025, offer step-by-step guidance for navigating these complex requirements.
For those seeking detailed information, the FDA's New Dietary Ingredient Notification Process page provides industry professionals and consumers with essential updates. Companies that successfully navigate the NDI process bring innovative, science-backed supplement new products to market, setting a benchmark for quality and safety.
Enforcement Actions and Consumer Protection
Enforcement actions are a defining feature of the supplement new regulatory environment. Between 2021 and 2025, the FDA has increased warning letters and product recalls, targeting supplements with unsubstantiated health claims, adulterated formulas, and improper use of novel ingredients. These actions reinforce the need for robust compliance and drive higher industry standards.
Consumer protection is also front and center. The FDA’s ingredient directory makes it easier to verify product legitimacy and spot red flags. Consumers should report adverse events and check supplement new products against official resources before use.
Table: Common Supplement Regulatory Violations (2021-2025)
| Violation Type | Example | Impact |
|---|---|---|
| Unsubstantiated Health Claims | “Cures disease” | Product recalls, fines |
| Adulterated Products | Undisclosed pharmaceuticals | Health risks, market removal |
| Novel Ingredient Misuse | Unapproved compounds | Legal action, warnings |
To stay safe, always read labels, consult healthcare providers, and use verified directories. With supplement new regulations evolving, these best practices empower both consumers and brands to make informed, responsible choices.
Ingredient Innovations: What’s New and Trending in 2025?
The supplement new landscape in 2025 is defined by a surge of innovative ingredients and advanced formulations. Driven by consumer demand for holistic wellness, the industry is moving rapidly to introduce compounds that are not only effective but also safe, transparent, and sustainable.

Emerging Ingredient Trends
This year, the supplement new market is witnessing an influx of novel ingredients supported by robust scientific research. Companies are investing in discovery, bringing forward lesser-known botanicals, marine extracts, and fermented bioactives. These advancements are expanding options for targeted health support.
- New marine-sourced omega-3s
- Advanced polyphenol complexes
- Rare adaptogenic herbs
Rigorous testing and transparent labeling are now standard, helping consumers trust supplement new products more than ever.
Scientific Validation and Regulatory Approvals
A defining feature of supplement new innovations in 2025 is their scientific validation. Regulatory agencies, such as the FDA, have introduced updated guidelines for ingredient safety and efficacy. Brands are conducting clinical trials and publishing data to support health claims.
Recently, the FDA issued guidance on live microbial agents and botanical safety, ensuring that supplement new formulations meet the highest standards. This emphasis on evidence is raising the bar for the entire industry.
Natural, Plant-Based, and Bioactive Compounds
Consumer interest in natural wellness is driving a shift toward plant-based and bioactive ingredients. Supplement new products now frequently feature botanicals, phytonutrients, and naturally derived compounds. These ingredients are chosen for their bioavailability and minimal processing.
Popular choices include:
- Turmeric and curcumin blends
- Plant-sourced antioxidants
- Superfood greens and reds
This trend reflects a broader movement toward clean-label, transparent supplement new formulations.
Adaptogens, Nootropics, and Gut Health
Adaptogens and nootropics have become central to supplement new development. Consumers are seeking products that not only support physical health but also cognitive function and stress resilience. The gut-brain axis is a major area of research, with new strains of probiotics and prebiotics entering the market.
- Ashwagandha and rhodiola extracts
- Lion’s mane and bacopa for cognition
- Synbiotic blends for digestive health
These supplement new categories are backed by emerging science and are increasingly tailored to individual needs.
Sustainable and Ethical Ingredient Sourcing
Sustainability is a core value in supplement new innovation. Companies are prioritizing ethically harvested, traceable ingredients to meet both environmental and consumer expectations. Advances in sourcing technology enable brands to verify the origin and purity of raw materials.
Many supplement new products now carry certifications for organic, fair-trade, or wildcrafted sourcing. This transparency fosters trust and aligns with consumer values.
Global Influences and Multi-Ingredient Blends
The supplement new industry is globalizing, with ingredient trends from Asia, Europe, and the Pacific influencing US and NZ markets. Multi-ingredient blends that target holistic health outcomes are gaining popularity, often combining adaptogens, antioxidants, and specialty nutrients in one formula.
For those seeking to understand why these innovations matter, consider the importance of nutraceuticals, which play a central role in the supplement new landscape.
As ingredient innovation accelerates, supplement new products are becoming more effective, sustainable, and tailored to diverse health goals. Staying informed will help consumers make empowered choices in 2025.
Advanced Supplement Delivery Systems and Formats
Innovation in supplement new delivery systems is redefining how people experience nutritional products in 2025. As the market expands, advanced technologies are making it easier for consumers to get maximum benefits from every dose. This shift is not just about what is in the capsule or powder, but how it gets delivered into the body for optimal results.
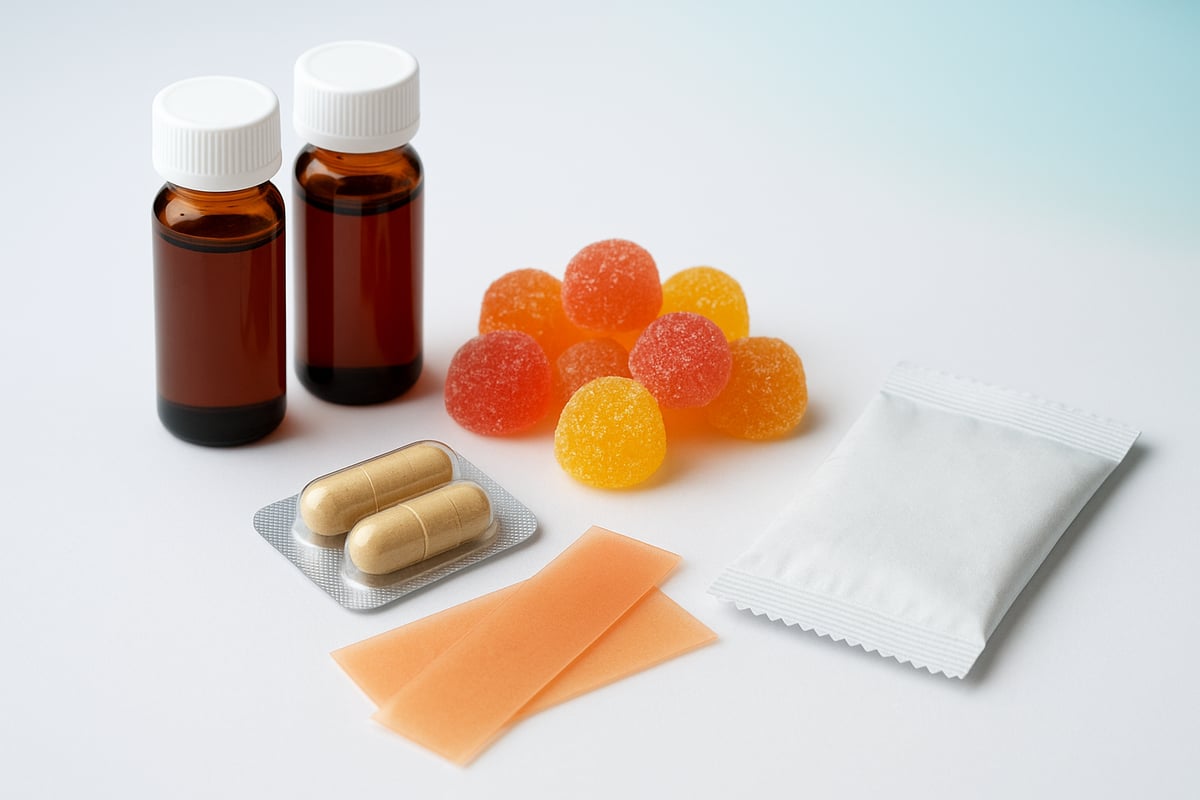
The Rise of Advanced Delivery Technologies
Recent years have seen a surge in supplement new technologies like liposomal encapsulation, nanoemulsions, and slow-release capsules. Liposomal delivery uses tiny fat-like particles to help nutrients pass through the digestive system more efficiently, leading to higher absorption rates. Nanoemulsions break down ingredients into microscopic droplets, making them easier for the body to use. Slow-release capsules are engineered to release active compounds over time, supporting sustained benefits such as extended nutrient availability or timed support for sleep.
These innovations are not just technical upgrades; they represent a response to consumer demands for products that are both effective and convenient. For example, time-of-day release supplements are now designed to target specific needs, such as supporting sleep at night or boosting energy in the morning. The supplement new landscape is thus becoming more tailored to individual routines.
Alternative Formats Reshape Supplement Experience
Consumers are increasingly drawn to alternative supplement new formats that blend function with enjoyment. Gummies, flavored powders, dissolvable strips, and ready-to-drink beverages have seen significant market share growth in 2024 and 2025. These formats appeal to those who want a pleasant taste, easy portability, and quick consumption.
The quest for better flavor is also driving technological breakthroughs. AI-powered platforms like TastePepAI: AI in Taste Peptide Design are now helping formulators create supplements with improved taste profiles, making daily routines more enjoyable. This kind of innovation is crucial as consumers expect their supplement new experience to fit seamlessly into their lifestyle, without the unpleasant aftertastes or inconvenience of traditional pills.
Consumer Preferences and Regulatory Considerations
The supplement new market is shaped not only by technology, but also by shifting consumer priorities. People now want supplements that are clean, transparent, and fit their personal wellness goals. Convenience and efficacy often go hand in hand, with consumers favoring products that offer both.
However, with rapid innovation comes the need for updated regulatory oversight. Agencies like the FDA are actively releasing new guidelines for supplement safety and novel delivery systems. For those interested in the latest compliance updates, the FDA's 2025 Human Foods Program Guidance Agenda outlines planned guidance documents that impact the supplement new industry, ensuring products remain both safe and effective.
As supplement delivery systems continue to evolve, both consumers and industry leaders must stay informed about the benefits, risks, and regulatory landscape. Advanced delivery formats are not just a trend, but a fundamental shift in how supplements support modern health and wellness.
Consumer Trends, Education, and Safety in 2025
Consumer awareness is reshaping the supplement new landscape in 2025. With the explosion of innovative products, people are seeking more than just promises on labels. They want proof, clarity, and resources to make informed decisions. This shift is driving unprecedented demand for transparency and education across the industry.
Consumer Education: The Foundation of Safe Choices
Regulatory agencies like the FDA are stepping up efforts to educate the public about supplement new products. In 2025, a wave of new educational materials is helping consumers understand what really goes into their supplements. These resources explain how to interpret labels, recognize legitimate certifications, and spot red flags.
For example, the FDA's updated directory of supplement ingredients offers a reliable way for consumers to verify what's inside their chosen supplement new products. This directory empowers users to check ingredient legitimacy before making a purchase.
Transparency and Trust: Sourcing, Testing, and Certification
Transparency is now a non-negotiable expectation among supplement new users. People want to know exactly where their ingredients come from, how products are tested, and which certifications back up quality claims.
Third-party testing and certifications are becoming key decision factors. Shoppers are looking for clear evidence of purity, potency, and safety. In fact, many leading brands now display certification logos on their packaging, making it easier for consumers to identify trustworthy supplement new options.
Table: Key Transparency Factors for Supplement New Products
| Factor | Why It Matters | What to Look For |
|---|---|---|
| Ingredient Sourcing | Ensures ethical, sustainable supply | Country of origin, harvest info |
| Third-party Testing | Confirms purity and potency | Independent lab results |
| Certifications | Validates safety and quality | NSF, USP, Informed Choice |
Reading Labels and Understanding Claims
The ability to read and interpret supplement new labels is essential for safety. Consumers must look beyond marketing promises and examine the Supplement Facts panel, ingredient lists, and certification details.
Be wary of products making unsubstantiated health claims or promising miracle results. Instead, focus on those with clear, evidence-based information. For those seeking additional guidance, resources like the Top 7 essential supplements highlight trending, reputable products to consider.
Online Resources, Reviews, and Social Proof
The digital age has put a wealth of supplement new information at everyone's fingertips. Online directories, community-driven reviews, and social media discussions help shoppers compare products and share real-world experiences.
Consumers are using these resources to validate claims, check for recalls, and report adverse events. Social proof, such as verified reviews or expert endorsements, can provide extra reassurance when choosing a supplement new product.
Best Practices for Supplement New Users
To maximize benefits and minimize risks, follow these best practices:
- Always consult a healthcare professional before starting any supplement new regimen.
- Use official resources like the FDA's ingredient directory or reputable third-party certification sites.
- Report any adverse reactions to regulatory authorities.
- Prioritize products with transparent sourcing, independent testing, and clear labeling.
- Stay informed by participating in educational programs and reading up on the latest trends.
By embracing these strategies, consumers can confidently navigate the supplement new market, making empowered choices that support their health and well-being.

